How
The following planning guide and economic tool were designed to help organizations decide how to implement routine tumor screening for Lynch syndrome in order to maximize success. The planning Guide may also help organizations decide whether to go straight to germline testing.
Economic Modeling Tool
Downloadable Excel Modeling Toolkit
Video showing how to use the tool
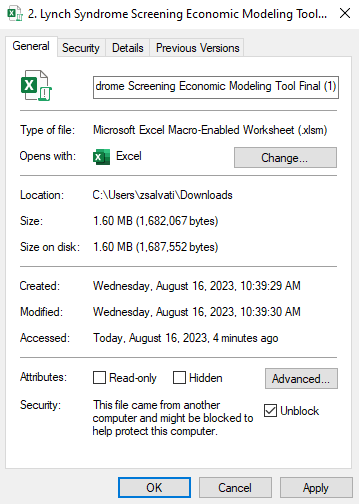
NOTE: when you download the Excel file you may need to disable macros by following the directions below:
Right-click on the file after you saved it to your computer, select “Properties”, and then click “Unblock” and “Apply” at the bottom of the General tab. From there all macros should be fully enabled.